Abstract
Background: Treatment options for patients (pts) with relapsed/refractory (r/r) AML and HR-MDS who cannot tolerate standard therapy are limited, and innovative combination approaches are needed. Dysregulation of the tumor protein 53 [p53, (TP53 gene)], Murine Double Minute-2 (MDM2) interaction leads to uncontrolled cell proliferation and tumor growth (Chene 2003). Siremadlin is an orally administered small molecule inhibitor of the p53-MDM2 interaction, which has a well-characterized safety profile and shows antitumor activity in pts with AML (Stein 2021). Combination of siremadlin with BCL-2 inhibitors (eg, venetoclax [VEN]) results in synergistic activity in p53 wild-type AML cell lines and leads to complete and durable antitumor responses in a variety of p53wt AML patient-derived xenograft models (Wang 2019). Thus, combined MDM2 and BCL-2 inhibition may result in improved outcomes for pts with hematologic malignancies. Here we report the preliminary results of a phase Ib, open-label study of siremadlin in combination with VEN for the treatment of pts with AML and HR- MDS (NCT03940352).
Methods: Pts were adults with r/r AML after ≥1 but ≤3 prior therapies who were not suited for standard therapy, or pts with AML who were unfit for standard chemotherapy. HR-MDS pts (per Revised International Prognostic Scoring System) who have previously failed hypomethylating agent therapy were also eligible. AML pts with TP53-mutant tumors (determined as the presence of any mutations in exons 5,6,7, and 8 at minimum) were excluded. Pts with prior treatment with an MDM2 or MDM4 inhibitor in combination with a BCL-2 inhibitor were excluded. Siremadlin (20 or 30 mg) was administered daily from day 1-5 during each 28-day cycle in combination with VEN at a starting dose of 50 mg daily, followed by a gradual ramp-up period over 4 days to a target daily dose of 400 mg (dose level [DL] 1: siremadlin 20 mg + VEN and DL2: siremadlin 30 mg + VEN]. Pts continued study treatment until unacceptable toxicity, disease progression, or investigator/patient decision. The primary objective is to evaluate safety/tolerability; secondary objectives are to test preliminary efficacy (per Cheson 2003) and pharmacokinetics (PK).
Results: As of data cutoff March 1, 2021, 18 total pts underwent treatment. The median age was 72.5 y (range, 29-84). Most pts had an Eastern Cooperative Oncology Group performance status of 0 (n=13, 72%). Of the 17 (94%) pts with AML, 3 pts had first-line and 14 pts had r/r AML. One (6%) pt had HR-MDS (DL1); efficacy data for this pt are not presented. Eleven pts were treated at DL1 and 7 at DL2. Treatment is ongoing for 2 pts (both DL2). The mean treatment duration was 3.8 mo (range, 0.9-10.3; DL1) and 1.8 mo (range, 0-3.8; DL2). Two pts (DL1) had ≥9 mo treatment duration.
The most common grade ≥3 adverse events (AEs) suspected to be treatment-related were neutropenia (n=5, 28%) and thrombocytopenia (n=4, 22%); 11 pts (61.1%) experienced grade ≥3 serious AEs regardless of study-treatment; only febrile neutropenia was experienced by >20% of pts (n=8, 44%). One case of tumor lysis syndrome was reported. Notably, gastrointestinal grade ≥3 serious AEs were uncommon (diarrhea [n=2, 11%] and nausea [n=1, 6%]).
Dose modification or interruption due to an AE occurred in 10 (56%) pts, mostly due to febrile neutropenia (n=4) and neutropenia (n=3), and discontinuation due to an AE occurred in 4 (22%) pts, mostly due to febrile neutropenia (n=2). Among the 11 pts in the dose-determining set, 2 experienced dose limiting toxicities (1 with anemia, bone marrow failure, and thrombocytopenia and 1 with febrile neutropenia). Two on-treatment deaths occurred; however, none were treatment-related.
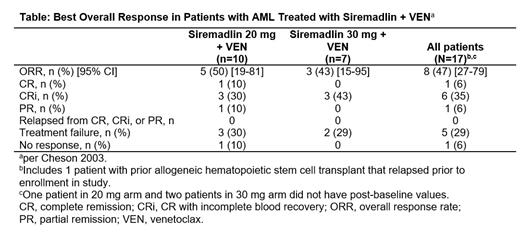
Among 10 pts with AML in DL1, 1 had complete remission (CR), 3 had CR with incomplete blood count recovery (CRi) and had 1 partial remission. Among 7 pts with AML in DL2, 3 pts had CRi (Table). One pt in DL2 currently has an ongoing CRi.
PK exposure and parameters for siremadlin and VEN for both cohorts were comparable to historical single-agent data. No drug-drug interaction between siremadlin and VEN was observed.
Conclusions: Siremadlin + VEN is well-tolerated in pts with AML and shows promising antileukemic activity in r/r AML pts. These results validate the MDM2-p53 interaction as a therapeutic target and provide support for further development of siremadlin + VEN in pts with AML or HR-MDS. Dose escalation is ongoing to determine the recommended dose for expansion.
Wei: Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Macrogenics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Breccia: Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Abbvie: Honoraria; Bristol Myers Squibb/Celgene: Honoraria. Cedena Romero: Janssen: Honoraria; BMS: Honoraria. Ciceri: IRCCS Ospedale San Raffaele: Current Employment. Erba: AbbVie Inc; Agios Pharmaceuticals Inc; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Incyte Corporation; Jazz Pharmaceuticals Inc; Novartis: Speakers Bureau; AbbVie Inc: Other: Independent review committee; AbbVie Inc; Agios Pharmaceuticals Inc; Astellas; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Daiichi Sankyo Inc; Genentech, a member of the Roche Group; GlycoMimetics Inc; Incyte Corporation; Jazz Pharmaceuticals Inc; Kura Oncology; Nov: Other: Advisory Committee; AbbVie Inc; Agios Pharmaceuticals Inc; ALX Oncology; Amgen Inc; Daiichi Sankyo Inc; FORMA Therapeutics; Forty Seven Inc; Gilead Sciences Inc; GlycoMimetics Inc; ImmunoGen Inc; Jazz Pharmaceuticals Inc; MacroGenics Inc; Novartis; PTC Therapeutics: Research Funding. Gaur: Novartis: Current Employment. Sechaud: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Halilovic: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Kuenzle: Novartis: Current Employment. Fabre: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.
Siremadlin is a small molecule inhibitor of the p53-MDM2 interaction under investigation for the treatment of patients with myeloid malignancies.